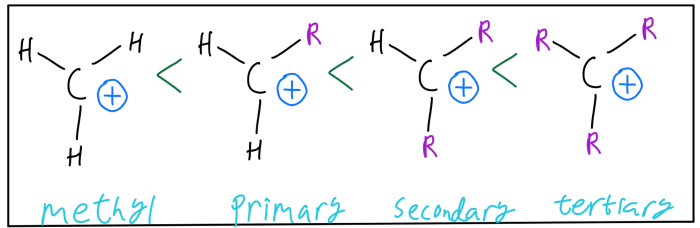
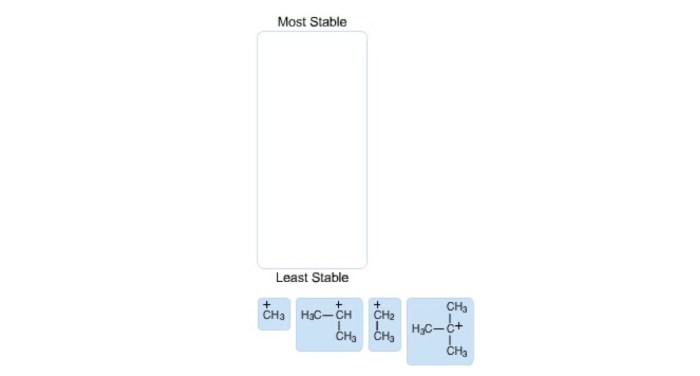
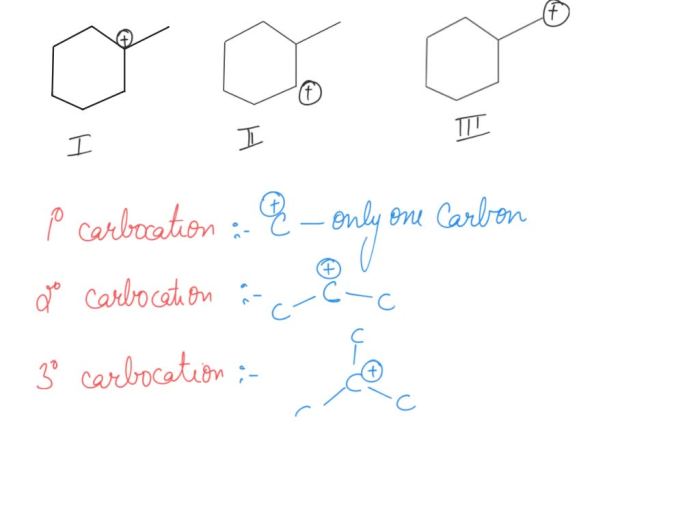
Rank the following carbocations in order of decreasing stability – Delving into the fascinating realm of carbocation stability, this discourse embarks on a journey to rank carbocations in order of their decreasing stability. Carbocations, positively charged carbon ions, exhibit varying degrees of stability based on their structural characteristics. Understanding these factors is crucial for comprehending the behavior and reactivity of carbocations in chemical reactions.
The stability of carbocations is primarily influenced by two key effects: inductive effects and resonance effects. Inductive effects arise from the electron-withdrawing or electron-donating nature of substituents attached to the carbocation-bearing carbon atom. Resonance effects, on the other hand, involve the delocalization of electrons over multiple atoms or bonds, leading to increased stability.
Carbocation Stability: Rank The Following Carbocations In Order Of Decreasing Stability
Carbocation stability is a measure of the relative reactivity of a carbocation. More stable carbocations are less reactive, and less stable carbocations are more reactive. The stability of a carbocation is determined by a number of factors, including:
- The number of alkyl groups attached to the carbocation
- The type of alkyl groups attached to the carbocation
- The presence of resonance
Carbocation stability increases with the number of alkyl groups attached to the carbocation. This is because alkyl groups are electron-donating, and they can help to stabilize the positive charge on the carbocation. The type of alkyl group attached to the carbocation also affects stability.
Primary alkyl groups are more electron-donating than secondary alkyl groups, which are more electron-donating than tertiary alkyl groups. This is because primary alkyl groups have more hydrogen atoms attached to them, and hydrogen atoms are electron-donating. Resonance can also help to stabilize carbocations.
Resonance is the delocalization of electrons over multiple atoms. When a carbocation is in resonance, the positive charge is spread out over multiple atoms, which makes the carbocation more stable.
Inductive and Resonance Effects
The inductive effect is the ability of a substituent to donate or withdraw electrons through sigma bonds. Electron-donating substituents increase the electron density on the carbocation, making it more stable. Electron-withdrawing substituents decrease the electron density on the carbocation, making it less stable.
The resonance effect is the ability of a substituent to donate or withdraw electrons through pi bonds. Electron-donating substituents increase the electron density on the carbocation, making it more stable. Electron-withdrawing substituents decrease the electron density on the carbocation, making it less stable.
Carbocation Rearrangements, Rank the following carbocations in order of decreasing stability
Carbocation rearrangements are reactions in which a carbocation rearranges to a more stable carbocation. Carbocation rearrangements are driven by the desire of the carbocation to achieve a more stable configuration. There are a number of different types of carbocation rearrangements, including:
- 1,2-shifts
- 1,3-shifts
- Wagner-Meerwein rearrangements
1,2-shifts are the most common type of carbocation rearrangement. In a 1,2-shift, a hydrogen atom or an alkyl group moves from one carbon atom to an adjacent carbon atom. 1,3-shifts are less common than 1,2-shifts. In a 1,3-shift, a hydrogen atom or an alkyl group moves from one carbon atom to a carbon atom that is two carbons away.
Wagner-Meerwein rearrangements are a type of 1,2-shift that occurs in carbocations that are formed from the addition of a proton to an alkene.
Carbocation Reactions
Carbocations can undergo a variety of reactions, including:
- Nucleophilic addition
- Electrophilic addition
- Elimination
- Rearrangement
Nucleophilic addition is the most common reaction of carbocations. In nucleophilic addition, a nucleophile attacks the carbocation, forming a new bond. Electrophilic addition is less common than nucleophilic addition. In electrophilic addition, an electrophile attacks the carbocation, forming a new bond.
Elimination is a reaction in which a carbocation loses a proton and an alkene is formed. Rearrangement is a reaction in which a carbocation rearranges to a more stable carbocation.
Commonly Asked Questions
What is the significance of carbocation stability in organic chemistry?
Carbocation stability is crucial in organic chemistry because it influences the reactivity and selectivity of carbocation-mediated reactions. Stable carbocations are less reactive and undergo rearrangements to form more stable species, while unstable carbocations are more reactive and participate in a wider range of reactions.
How can we predict the stability of a carbocation?
The stability of a carbocation can be predicted based on the inductive and resonance effects of substituents attached to the carbocation-bearing carbon atom. Electron-withdrawing substituents increase carbocation stability by withdrawing electrons from the positive carbon, while electron-donating substituents decrease stability by donating electrons.