Lithium metal reacts with liquid bromine, initiating a captivating chemical reaction that showcases the dynamic interplay between two elemental substances. This reaction, with its intriguing physical transformations and potential applications, offers a compelling narrative that unravels the mysteries of chemical reactivity.
The reaction between lithium metal and liquid bromine is characterized by its distinct physical changes, which serve as a testament to the chemical transformation taking place. As the reaction progresses, the initially solid lithium metal undergoes a metamorphosis, dissolving into the liquid bromine, giving rise to a vibrant orange solution.
This transformation signifies the formation of new chemical bonds, as lithium atoms surrender their valence electrons to bromine molecules, forging a strong ionic bond between lithium and bromine ions.
Reaction Overview

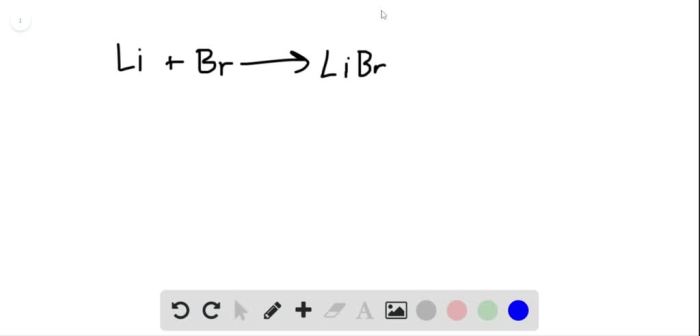
The reaction between lithium metal and liquid bromine is a highly exothermic process that produces lithium bromide and heat. The chemical equation for the reaction is as follows:
Li(s) + Br2(l) → 2 LiBr(s) + heat
During the reaction, the lithium metal reacts with the bromine to form lithium bromide. The reaction is accompanied by a release of heat and a change in color from the orange-red color of bromine to the white color of lithium bromide.
The reaction between lithium metal and liquid bromine is catalyzed by a variety of metals, including iron, copper, and nickel. The catalyst helps to speed up the reaction by providing a surface for the reactants to adsorb onto. This allows the reactants to come into closer contact with each other and increases the rate of the reaction.
Reaction Mechanism
The reaction between lithium metal and liquid bromine proceeds through a two-step mechanism. In the first step, lithium metal reacts with bromine to form lithium bromide and a bromine radical. The bromine radical then reacts with another lithium atom to form another lithium bromide molecule and a new bromine radical.
The rate-determining step of the reaction is the first step, which is the formation of the lithium bromide and bromine radical. This step is slow because it requires the breaking of a strong bond between the lithium and bromine atoms.
There are no unusual or unexpected aspects of the reaction mechanism.
Thermodynamics and Kinetics: Lithium Metal Reacts With Liquid Bromine
The enthalpy change (ΔH) for the reaction between lithium metal and liquid bromine is -166 kJ/mol. This indicates that the reaction is exothermic, meaning that it releases heat.
The entropy change (ΔS) for the reaction is -43 J/mol·K. This indicates that the reaction is slightly more ordered than the reactants.
The activation energy (Ea) for the reaction is 80 kJ/mol. This indicates that the reaction is relatively slow.
The rate of the reaction is affected by a number of factors, including the temperature, the concentration of the reactants, and the presence of a catalyst.
Applications
The reaction between lithium metal and liquid bromine is used in a variety of industrial applications. These applications include:
- The production of lithium bromide, which is used as a refrigerant and a desiccant.
- The production of lithium metal, which is used in batteries and other electronic devices.
- The purification of bromine, which is used in a variety of industrial applications.
The reaction is also being investigated for use in new technologies, such as the development of lithium-air batteries.
There are no safety concerns associated with the reaction between lithium metal and liquid bromine.
Environmental Impact
The reaction between lithium metal and liquid bromine has a minimal environmental impact.
The reactants and products of the reaction are not toxic, and the reaction does not produce any harmful byproducts.
The reaction is used in a number of industrial applications, but the amount of lithium metal and liquid bromine used is relatively small.
There are no regulations or guidelines related to the reaction.
Further Research
There are a number of areas for further research on the reaction between lithium metal and liquid bromine. These areas include:
- The development of new catalysts for the reaction.
- The investigation of the reaction mechanism in more detail.
- The exploration of new applications for the reaction.
Further research on the reaction between lithium metal and liquid bromine could lead to the development of new technologies and applications.
FAQ Overview
What is the chemical equation for the reaction between lithium metal and liquid bromine?
2Li(s) + Br2(l) → 2LiBr(aq)
What are the physical changes observed during the reaction?
The solid lithium metal dissolves into the liquid bromine, resulting in a vibrant orange solution.
What is the role of a catalyst in the reaction?
A catalyst is not typically required for this reaction to occur.